Shaw, B. et al. (2023). ‘Preservation of whole antibodies in ancient teeth’, iScience, 26(9), pp. 1-11
Background
Palaeoproteomics, or the study of ancient proteins, is an emerging field that intersects palaeontology, archaeology and biochemistry. Its extension into the field of dentistry and oral bioscience has remained notably limited, until now. This study reveals that teeth obtained from the Chester Greyfriars excavation site (dating between 1285-1470AD) contained functioning, disulphide-linked antibodies. Only some proteins, such as the structural protein collagen, were thought to be stable for such a long period of time.
Method
Researchers conducted proteomic analyses on the excavated teeth from specimens believed to have had Paget’s disease, rheumatoid arthritis, and osteoporosis. The proteins were firstly analysed using liquid chromatography tandem MS (LC-MS/MS) for protein sequencing. In order to purify the antibodies, the researchers applied a ‘Protein G-based affinity purification’ method. To measure the antibodies’ immunoreactivity, western blots were conducted using the purified ancient antibodies against a contemporary Epstein-Barr virus antigen.
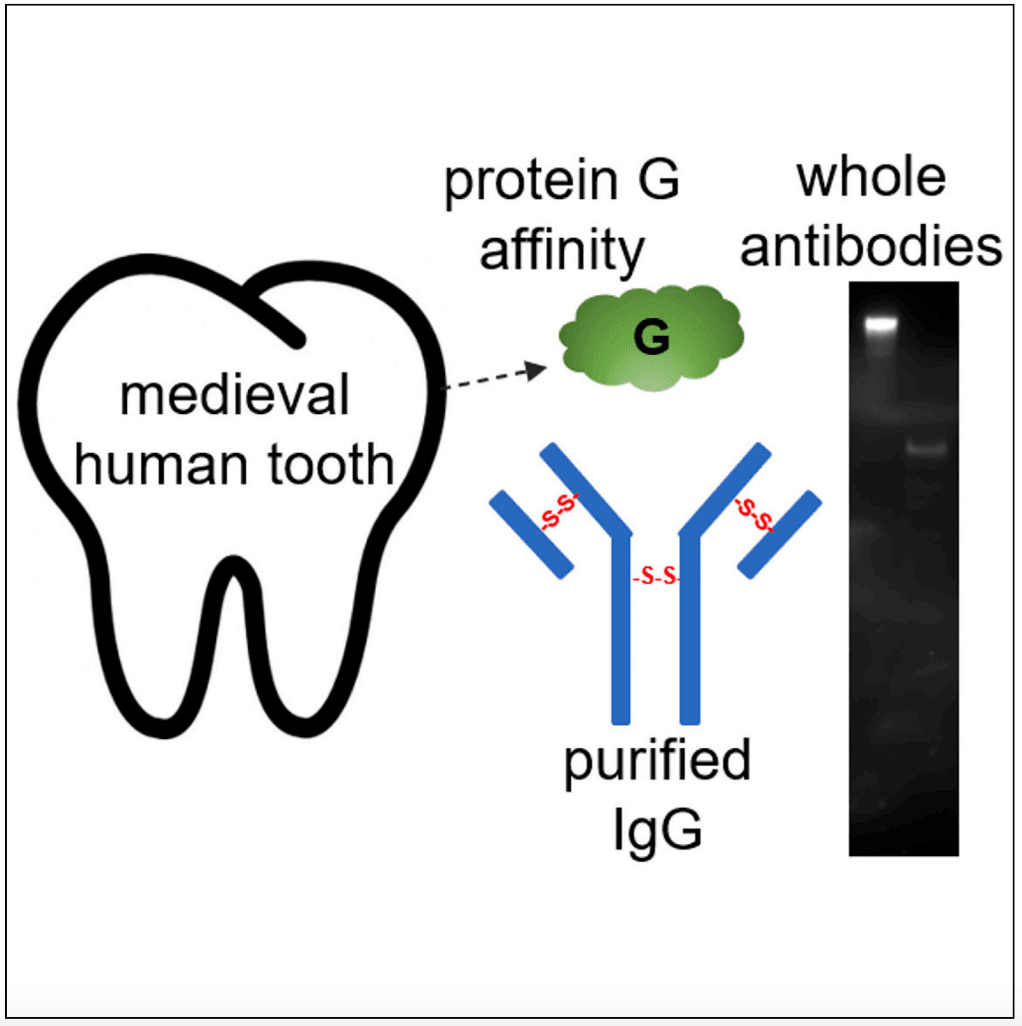
Figure 1: A Visualisation of the extraction of protein G, purified IgG, and the migration of bands on the Western Blot.
Results
After decalcifying the specimens, researchers found that complex protein mixtures could be successfully extracted from the ancient teeth. Specifically, low-level human antibody (IgG) peptide sequences were detected in the phosphate-soluble fractions. Further, the G-based affinity purification method produced purified proteins from phosphate-soluble extracts, with the Fc regions remaining intact. Perhaps the most significant finding was that the purified ancient antibodies retained their disulphide linkages. Under ‘reducing’ conditions (conditions designed to break the disulphide links), the antibodies exhibited a change in band migration on the Western blots. This change indicated that the antibodies remained intact, thus preserving their structural integrity. Finally, the ancient antibodies retained their immunoreactivity, even against contemporary pathogens. Using an Epstein-Barr virus, the researchers demonstrated a clear cross-reactivity. Specifically, antibodies from osteoporotic and rheumatoid arthritic specimens’ teeth reacted with antigens from a purified recombinant ‘His-EBNA-1’ protein. As such, the ancient antibodies were immunoreactive against ‘contemporary’ antigens found in present-day organisms. In this regard, the antibodies may be able to indicate the ‘molecular memory’ of a person’s immune status at the time of death.
Limitations
- Only a small sample of teeth were studied, decreasing the generalisability of the findings.
- The ‘whole tooth’ was purified, not the separate components. Future research could purify antibodies from enamel, dentin and the root independently to see where in the tooth the antibodies originated.
Conclusion
Ancient teeth can harbour functioning antibodies that not only retain their disulphide links, but that also exhibit immunoreactivity against contemporary antigens, such as a present-day Epstein-Barr virus. The antibodies could be sequenced with liquid chromatography tandem MS (LC-MS/MS), and purified with G-based affinity purification successfully. Further, the study suggests that non-structural proteins, such as antibodies, can retain their functionality over such a long period. It lays a strong foundation for future study of the efficacy of teeth in preserving biological molecules through time.
Research Summary Written By: John Daly, University of Manchester – BDS1
