Janine Doughty, Fiona Burns, Anthony J. Santella, Aidan G. O’Keeffe, Stephen Porter and Richard G. Watt British Dental Journal (2025) Online Publication
Aim:
Assess the acceptability and feasibility of point of care testing (POCT) for Human Immunodeficiency Virus (HIV)in a dental setting in order to undertake a trial.
Background:
The rate of new diagnoses of HIV have increased in the UK by 22%. This increase was found in all population groups. With the invention of successful antiretrovirals therapy (ART), an undetectable viral load means that HIV cannot be transmitted. ARTs do not cure HIV, but can allow patients with HIV to experience a normal life expectancy with significant reduction in symptoms. If the CD4+ count is above 200 cells/mm3 when ART is initiated, life expectancy increases and health complications decrease significantly.
45% of HIV diagnoses were made when CD4+ is <350 cells/mm3 or when the disease manifests to AIDs within 3 months of diagnosis (late stage). Diagnoses in the late stage increase the risk of death by around ten times and can reduce life expectancy.
Methods:
A systematic review was undertaken, including similar studies from North America. Focus groups involving dental professionals and patients exploring how the POCT design can fit within the UK dental setting were done.
Table one shows the interventions used in this study. The INSTI capillary blood-based HIV-1/HIV-2 rapid antibody test was the POCT of choice.
Benefits of INSTI include: results are provided in one minute, 99.6% sensitivity, 99.3% specificity.
The HIV POCT was offered to patients verbally using an opt out system and testing took place after treatment or during any breaks. Results of the test were given within the same appointment. POCT testing was offered to patients meeting the inclusion criteria which included:
- Patients aged 18 years and over who attended during the study period
- Patients with sufficient understanding of English in order to provide consent
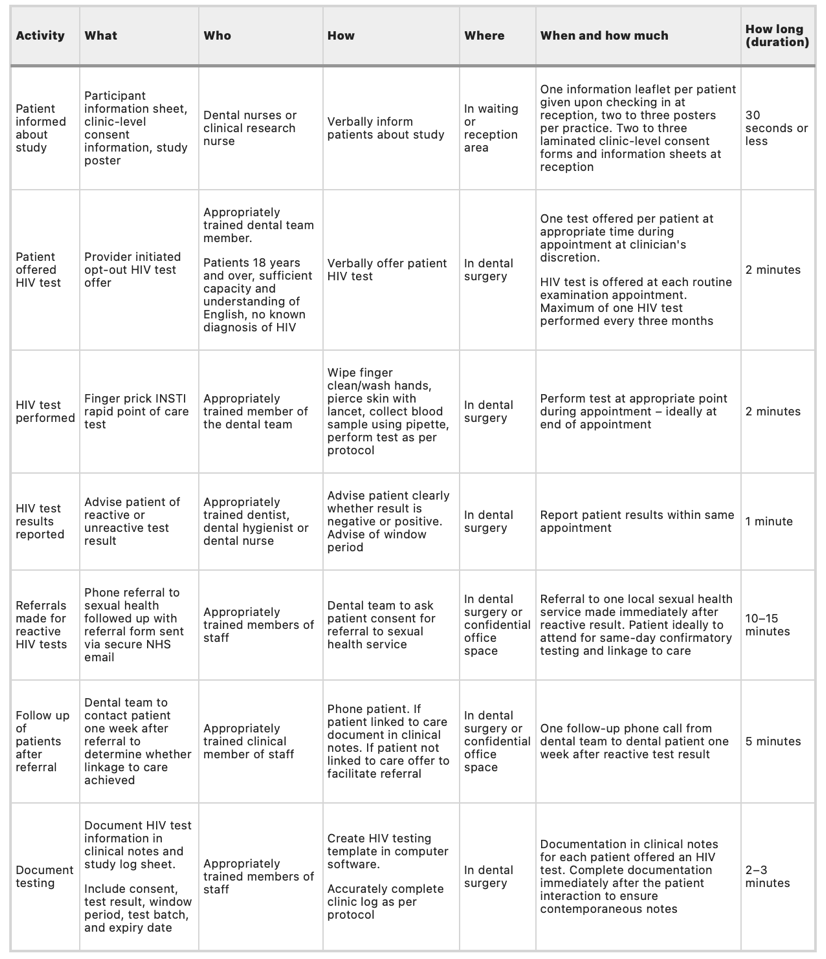
Table 1
Exclusion criteria included:
- Patients lacking capacity to consent to HIV testing
- Patients who couldn’t provide consent due to lack of comprehension of English
- Patients with a known diagnosis of HIV
- Patients who have had HIV POCT at the same place within 3 months
For patients that tested positive for HIV, referral pathways were developed with local sexual health services (SHS) to manage these diagnoses. This pathway involved a phone call to the SHS, followed by a referral that was sent securely via email. Consent was required for the referral.
The sample size included 441 patients with an 85% confidence interval. Two community dental services, one private dental practice and one NHS dental practice were included in the recruitment of patients. These four dental services were based in central London in areas that have high prevalence of HIV.
Results:
211 patients accepted the HIV test, resulting in 210 negative results and one positive result. The positive result came from a patient who did not disclose a known HIV diagnosis. A higher proportion of male patients accepted the POCT than females, however there were no significant associations between gender and the likelihood to accept testing.
POCT acceptance in the community dental services and NHS practice was higher than in private practice.
Conclusions:
A full scale trial in the UK would not be appropriate. The intervention had too many challenges that prevent a full-scale trial in the UK. These challenges included cost, recruitment and issues stated by patients and dental professionals. The referral pathway was not tested as no disease was found among participants, therefore staff acceptability on that side could not be verified.
Research Summary Written By: Omobolaji Adenuga, University of Manchester – BDS 4
